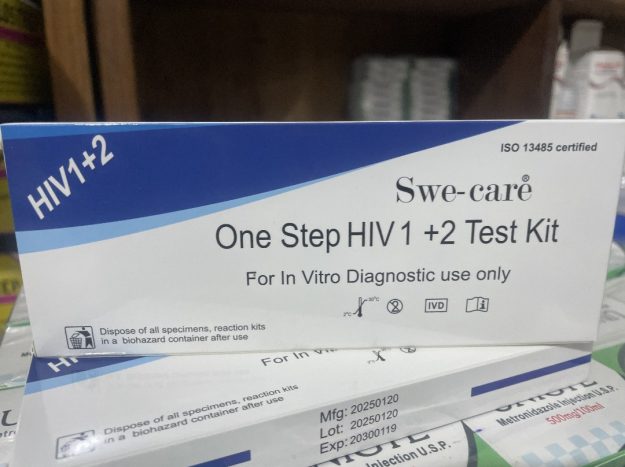
The One Step HIV 1+2 Test Kit is a qualitative, immunochromatographic assay designed for the professional detection of antibodies to both Human Immunodeficiency Virus Type 1 (HIV-1) and Type 2 (HIV-2). It is intended for use as a screening tool by healthcare professionals.
Key Features and Specifications:
Purpose: To provide a rapid, preliminary result for HIV infection. It is important to note that reactive results must be confirmed with additional, more specific supplemental tests (like a Western blot or PCR).
Target: Detects antibodies to HIV-1 and HIV-2.
Format: A one-step, lateral flow test strip, which means the procedure is simple and provides results quickly, typically within minutes.
Certification: The product is manufactured under the ISO 13485 quality management standard, which is specific to medical devices and ensures consistent design, production, and distribution.
Manufacturer: The kit is produced by Swe-care.
Intended Use: Strictly For In Vitro Diagnostic Use Only, meaning it is used outside the body on collected samples in a controlled setting.
Safety: The packaging includes clear warnings to dispose of all used test components and specimens in a biohazard container to ensure safe handling and prevent contamination.
Lot Information: The manufacturing date (Mfg), lot number (Lot), and expiration date (Exp) are provided for quality control and traceability. This specific kit was manufactured on January 20, 2025, and expires on January 19, 2030.








Reviews
There are no reviews yet.