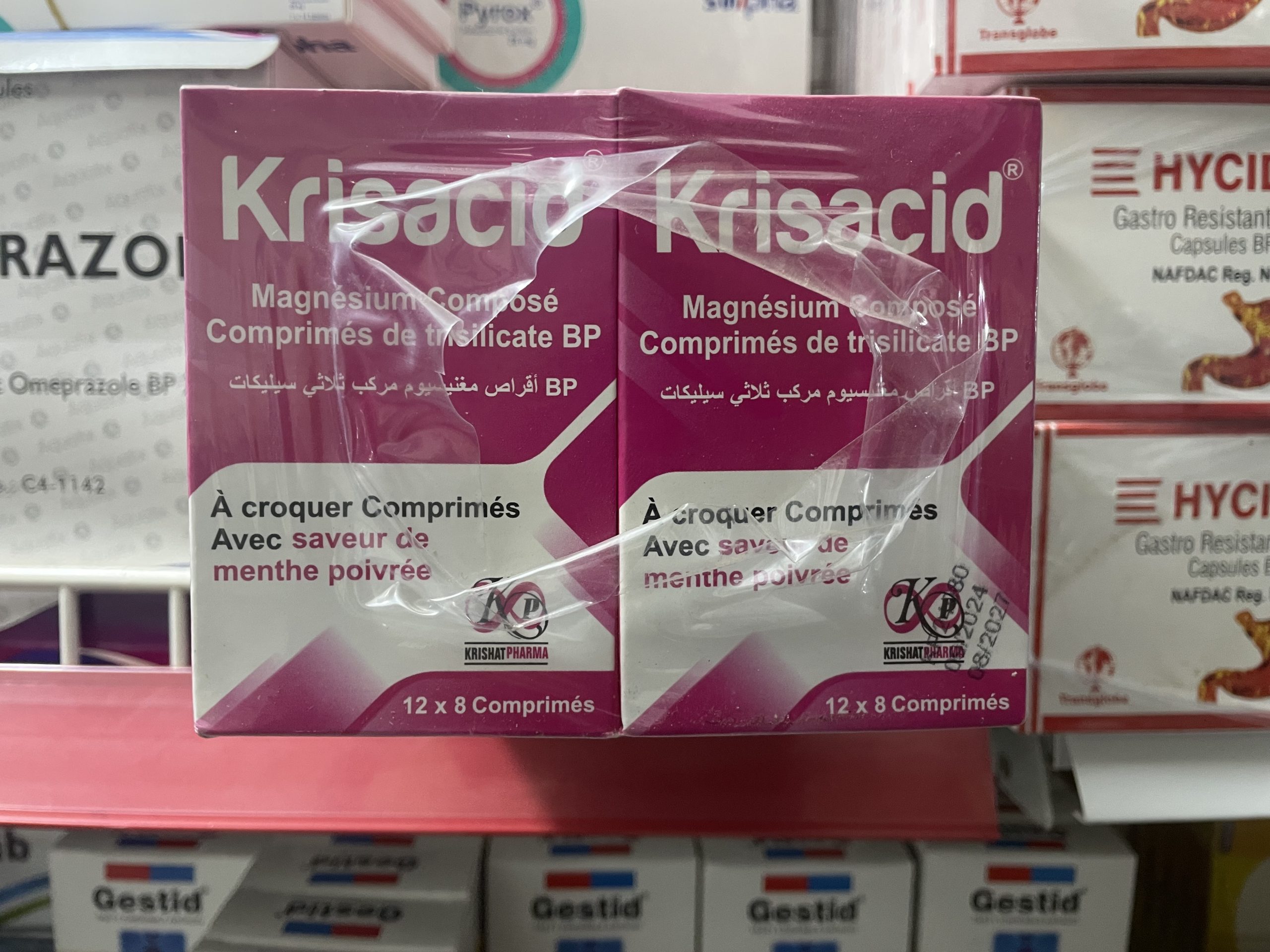
What is Krisacid?
Krisacid is an antacid product manufactured by Krishat Pharma Industries Limited, located on the Lagos-Ibadan Expressway, Nigeria. It’s registered with NAFDAC.
There are two common forms:
-
Krisacid Suspension (liquid form), each 5 mL contains Magnesium trisilicate BP 250 mg, Light Magnesium Carbonate BP 250 mg, Sodium Bicarbonate BP 250 mg.
-
Krisacid Chewable Tablets (tablet form), which contain Magnesium trisilicate 250 mg + Aluminum hydroxide (dried) 120 mg with peppermint flavor.
Indications (Uses):
Krisacid is used for relief of symptoms of:
-
Heartburn
-
Hyperacidity
-
Dyspepsia (indigestion)
-
Acid reflux
-
Gas, bloating, sour stomach
Dosage & How to Use:
-
For adults and children over 12 years: 2-4 spoonfuls (5 mL each) of suspension, taken up to three times per day or as needed.
-
For children 5–12 years: 1-2 spoonfuls (5 mL each), up to three times daily.
-
For tablets: chew thoroughly; follow package instructions and/or physician advice when to take.
-
After taking tablets or suspension, having a little water may help, especially with suspension. Shake suspension before use.
Contraindications & Precautions:
-
Do not use in severe renal failure, hypophosphataemia
-
Avoid if you must restrict sodium intake (e.g. in hypertension, congestive heart failure) given sodium bicarbonate content.
-
Use with care in patients with metabolic or respiratory acidosis, hypocalcaemia, or hypochlorhydria. NAFDAC Greenbook
-
Do not administer to children under 5 years unless directed by a physician. NAFDAC Greenbook
-
If symptoms persist beyond a certain time, consult a doctor. NAFDAC Greenbook
Side Effects:
-
Mild effects: flatulence, stomach cramps, diarrhea may occur due to magnesium components.
-
Overuse or overdose: risk of hypermagnesaemia, metabolic alkalosis, sodium overload. Signs may include confusion, muscle weakness, etc.
Storage & Regulatory Info:
-
Store in a cool, dry place. Once opened, suspension should be used within 28 days.
-
Manufacturer: Krishat Pharma Industries Limited, Ibadan, Oyo State, Nigeria.
-
Legal Status: Over-the-Counter (OTC) for many forms (chewable tablets, suspension). However the suspension is listed in NAFDAC green book.










Reviews
There are no reviews yet.